Introduction: FLT3-Internal Tandem Duplication (FLT3-ITD) mutations of the gene encoding the FLT3 tyrosine kinase receptor are found in 25-30% of AML patients and are associated with a poor prognosis despite intensive chemotherapy and allogeneic hematopoietic stem cell transplantation (HSCT). FLT3 tyrosine kinase inhibitors (TKI) significantly improve survival of FLT3 mutated AML patients in large prospective trials (Stone, NEJM 2017; doi:10.1056/NEJMoa1614359). Post-HSCT maintenance with sorafenib - a broad-spectrum TKI with strong activity against FLT3 - was recently shown to reduce the risk of relapse and improve survival in the phase II SORMAIN trial (Burchert, JCO 2020; doi: 10.1200/JCO.19.03345). Importantly, clinical trials reported a low rate of toxicities requiring sorafenib discontinuation (Chen et al, BBMT 2014;doi: 10.1016/j.bbmt.2014.09.007; Burchert et al., J Clin Oncol. 2020; doi: 10.1200/JCO.19.03345). The aim of our single-center retrospective analysis was to evaluate the real-life experience in patients treated with post-allogeneic HSCT sorafenib maintenance therapy for FLT3-ITD AML with a particular focus on tolerability and toxicity-related treatment interruption.
Methods: We conducted a single-center retrospective study on 33 FLT3-ITD AML patients undergoing allogeneic HSCT in complete remission between 2013 and 2020, including 20 patients who received sorafenib maintenance and 13 who did not. Sorafenib was started at hematologic reconstitution at 200 mg BID and increased at 400 mg BID after a week in case of good tolerance. Treatment was maintained until two years if tolerated. In case of toxicity, the drug was either reduced to 200mg BID, or stopped. Among the 13 patients in the untreated group, 8 were transplanted between 2013 and 2016 before we started to routinely use sorafenib maintenance, and 5 were transplanted in 2017 or later. Among the latter, sorafenib maintenance was not started because of aGVHD and insufficient hematologic reconstitution (n=3), low-allelic ratio of FLT3-ITD (n=1), early death due to PTLD (n=1).
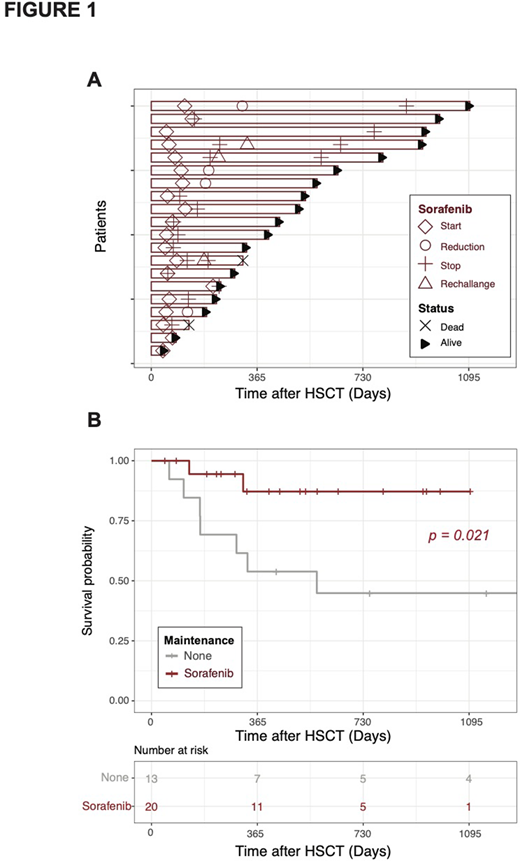
Results: In the sorafenib group (n=20), median time from transplant to sorafenib initiation was 68 days (range 40-213). Overall, 16 patients (80%) experienced toxicities leading to dose reduction (4 patients, 20%) or interruption (12 patients, 60%). In these patients, the average time on sorafenib before toxicity occurrence was 98 days (range 1-717; only one patient stopped treatment after 1 day because of severe diarrhea). Most common toxicities were skin (7, grade II), gastrointestinal (5, grade II), and hematologic (5, grade II and III). One patient experienced uveitis (grade III) and pneumonia (grade IV), both resolutive after sorafenib interruption. One patient experienced severe hypertension (grade III), 1 had hepatitis (grade III) and another one a PRESS syndrome (grade IV) possibly related to sorafenib.
Other causes of sorafenib interruption included FLT3-ITD negative relapse in 1 patient, relapse in 1 patient, and end of scheduled maintenance in 1 patient. Among the 12 patients who interrupted sorafenib because of toxicities, 3 patients were re-challenged with good tolerance in 2 cases, while 7 were switched to midostaurine. Among these 7, 4 completed the 2-y treatment and 3 interrupted midostaurine because of toxicities (2 patients experienced digestive intolerance and 1 pneumonia).
The average time of sorafenib exposure in the whole sorafenib group was 180 days (range 1-765). Importantly, the analysis of patients' outcome confirmed the previously reported positive impact of sorafenib maintenance on overall survival (Figure 1B) despite high rates of treatment interruption.
Conclusion: Our real-life analysis reveals higher rates of toxicity-related interruption of sorafenib maintenance after allogeneic HSCT than previously reported in clinical trials. Interestingly, our results confirm the favorable impact of sorafenib maintenance on clinical outcome despite a high rate of toxicity-related treatment interruption, suggesting the feasibility of switching to other maintenance approaches in case of low tolerability.
No relevant conflicts of interest to declare.
sorafenib as a maintenance treatment to prevent FLT3-ITD mutated AML reccurence after allogeneic transplantation
Author notes
Asterisk with author names denotes non-ASH members.